Gần đây, Cục Giám sát thị trường Gia Hưng đã tiến hành lấy mẫu toàn diện nước xử lý của Công ty TNHH Thiết bị y tế Haiyan Kangyuan và công bố rằng nước xử lý của Kangyuan Medical hoàn toàn đáp ứng các yêu cầu về nước tinh khiết của Dược điển Trung Quốc năm 2020, chứng minh năng lực kiểm soát tuyệt vời của Kangyuan Medical về chất lượng sản phẩm và an toàn cho bệnh nhân.
Việc kiểm tra mẫu do Cục Quản lý Thị trường Gia Hưng tổ chức và được Viện Kiểm định và Kiểm nghiệm Chất lượng Thực phẩm, Dược phẩm và Sản phẩm Gia Hưng ủy quyền. Theo các tiêu chuẩn quốc gia và quy chuẩn ngành liên quan, Viện Kiểm định và Kiểm nghiệm đã tiến hành kiểm tra toàn diện và chuyên nghiệp đối với nước xử lý mà Kangyuan Medical sử dụng để sản xuất các thiết bị y tế, bao gồm độ pH của nước, nitrat, độ dẫn điện, kim loại nặng, giới hạn vi sinh vật và nhiều chỉ tiêu khác. Sau nhiều vòng kiểm tra nghiêm ngặt, kết quả cho thấy nước xử lý của Kangyuan Medical hoàn toàn đáp ứng các yêu cầu về nước tinh khiết theo Dược điển Trung Quốc phiên bản 2020, đảm bảo tuyệt đối về chất lượng, an toàn và độ tin cậy của các sản phẩm thiết bị y tế Kangyuan.
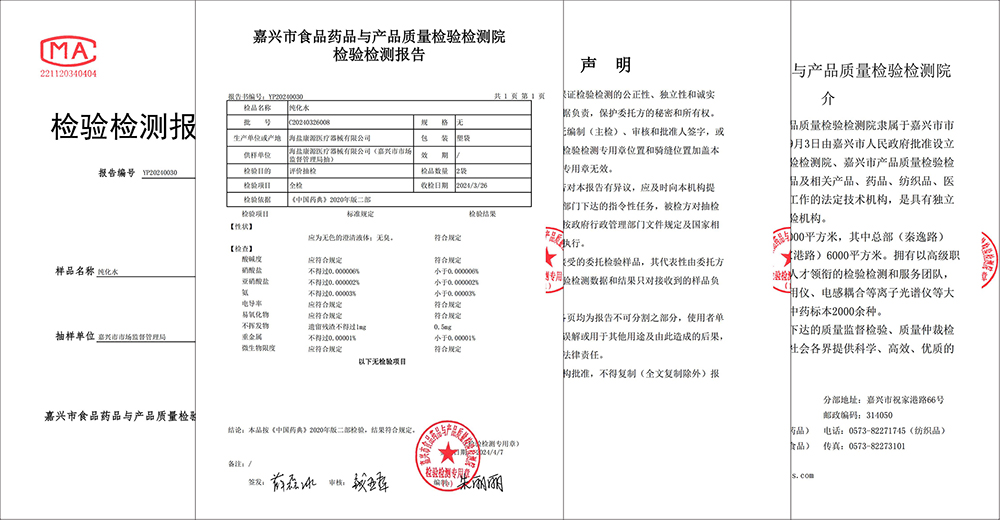
Kangyuan Medical luôn đặt chất lượng sản phẩm và an toàn cho bệnh nhân lên hàng đầu, đặc biệt coi trọng việc kiểm soát chất lượng nước xử lý. Công ty đã đầu tư thiết bị sản xuất nước và công nghệ giám sát tiên tiến, đồng thời thiết lập hệ thống quản lý nước xử lý và quy trình vận hành hợp lý, đảm bảo từng giọt nước đều đạt tiêu chuẩn quốc gia. Việc vượt qua đợt kiểm tra lấy mẫu không chỉ khẳng định chất lượng nước xử lý của Kangyuan Medical mà còn là sự công nhận cho toàn bộ hệ thống quản lý chất lượng của Kangyuan Medical.
Trong tương lai, Kangyuan Medical sẽ duy trì sự tích lũy sâu rộng trong ngành và tinh thần đổi mới liên tục, tiếp tục đóng vai trò dẫn đầu trong ngành y tế, đảm bảo cho phần lớn bệnh nhân được tiếp cận với vật tư y tế chất lượng tốt hơn và an toàn hơn, đóng góp nhiều hơn cho sự nghiệp chăm sóc sức khỏe con người.
Thời gian đăng: 29-05-2024

 中文
中文